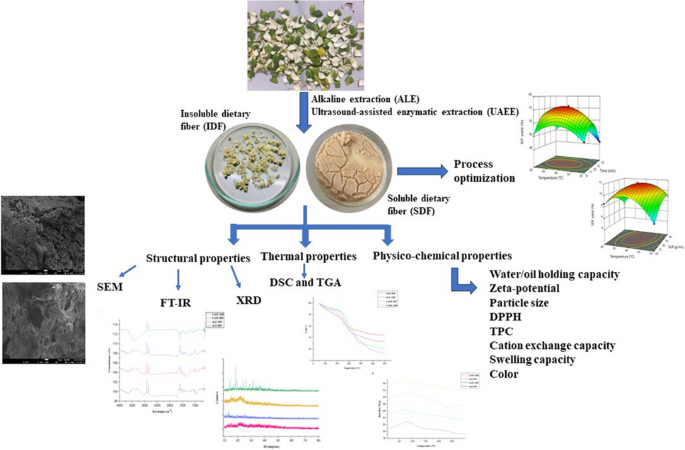
Green valorization approach of Citrus limetta peels by ultrasound-assisted enzymatic extraction for recovery of dietary fibers: Optimization, physicochemical, structural, functional, and thermal properties

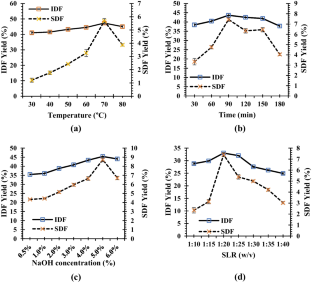
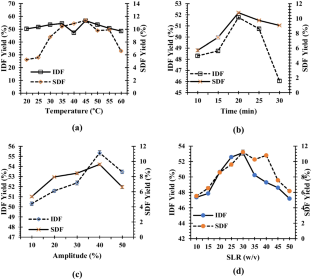
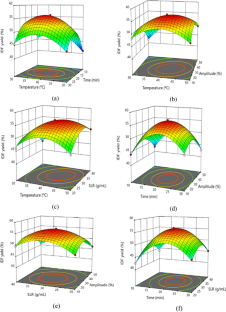
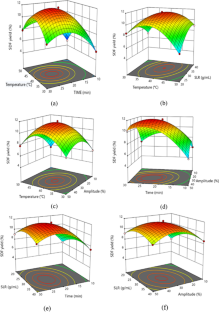
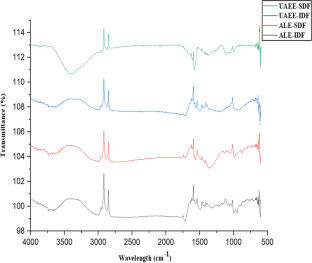
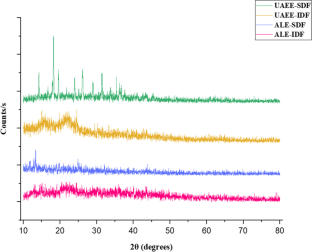
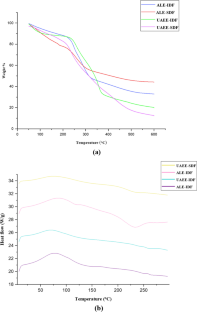
Valorization of citrus by-products for the extraction of dietary fibers can help neutralize increasing environmental issues and expedite their value-addition and sustainable applications, thereby promoting circular economy approach. In the present study, dietary fibers were recovered from Citrus limetta peels using green ultrasound-assisted enzymatic extraction (UAEE) technique and response surface methodology (RSM) was utilized to optimize the extraction process. The experimental yield value (insoluble dietary fiber = 55.97% and soluble dietary fiber = 11.83%) was close to the predicted yield value (insoluble dietary fiber = 55.89 ± 0.46% and soluble dietary fiber = 12.04 ± 0.21%) under the optimal conditions of 21.5 min sonication time, 30 v/w solid–liquid ratio, temperature 41 °C, and 31% amplitude, thus confirming the validity of the model. In addition, various proximate, functional, thermal, structural, and antioxidant properties of fibers recovered using both techniques were investigated and compared. Higher TPC, DPPH scavenging activity, water/oil holding, cation exchange, and swelling capacities of UAEE extracted fibers as compared to ALE fibers. Moreover, thermal, XRD, and SEM analysis showed that dietary fiber extracted using UAEE exhibited higher thermal stability and crystalline nature as well as showed loose, porous, and wrinkled surface than ALE-extracted fibers, respectively. In addition, difference in peak positions and intensities for characteristic bands of all fiber samples were observed. Therefore, these results suggested that UAEE can be used as a promising green extraction technique for the recovery of dietary fibers from C. limetta peels.
Graphical Abstract
This is a preview of subscription content, log in via an institution to check access.
Access this article
Subscribe and save
Springer+ Basic
€32.70 /Month
- Get 10 units per month
- Download Article/Chapter or eBook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime
Buy Now
Price includes VAT (France)
Instant access to the full article PDF.
Rent this article via DeepDyve

Similar content being viewed by others
Extraction and evaluation of structural and physicochemical properties of dietary fiber concentrate from mango peels by using green approach
Article 10 July 2021
Extraction and characterization of ultrasound assisted extraction: improved functional quality of pectin from jackfruit (Artocarpus heterophyllus Lam.) peel waste
Article 14 September 2023

Response surface optimization, kinetic modeling, and thermodynamic study for ultrasound-assisted extraction of dietary fiber from mango peels and its structural characterization
Article 18 April 2024
Explore related subjects
References
- Satari B, Karimi K (2018) Citrus processing wastes: environmental impacts, recent advances, and future perspectives in total valorization. Resour Conserv Recycl 129:153–167 ArticleGoogle Scholar
- Panwar D, Panesar PS, Chopra HK (2022) Green extraction of pectin from Citrus limetta peels using organic acid and its characterization. Biomass Convers Biorefinery 14:1–13 Google Scholar
- Buyukkurt OK, Guclu G, Kelebek H, Selli S (2019) Characterization of phenolic compounds in sweet lime (Citrus limetta) peel and freshly squeezed juices by LC-DAD-ESI-MS/MS and their antioxidant activity. J Food Meas Charact 13:3242–3249 ArticleGoogle Scholar
- Panwar D, Panesar PS, Chopra HK (2021) Recent trends on the valorization strategies for the management of citrus by-products. Food Rev Intl 37(1):91–120 ArticleGoogle Scholar
- Fuller S, Beck E, Salman H, Tapsell L (2016) New horizons for the study of dietary fiber and health: a review. Plant Foods Hum Nutr 71:1–12 ArticleGoogle Scholar
- He Y, Wang B, Wen L, Wang F, Yu H, Chen D, Su X, Zhang C (2022) Effects of dietary fiber on human health. Food Sci Human Wellness 11(1):1–10 ArticleGoogle Scholar
- FDA (2020) Food labeling: revision of the nutrition and supplement facts Labels: Guidance for Industry https://www.fda.gov/FoodGuidances
- Moczkowska M, Karp S, Niu Y, Kurek MA (2019) Enzymatic, enzymatic-ultrasonic and alkaline extraction of soluble dietary fibre from flaxseed–a physicochemical approach. Food Hydrocoll 90:105–112 ArticleGoogle Scholar
- Dhingra D, Michael M, Rajput H, Patil RT (2012) Dietary fibre in foods: a review. J Food Sci Technol 49:255–266 ArticleGoogle Scholar
- Beretta MV, Bernaud FR, Nascimento C, Steemburgo T, Rodrigues TC (2018) Higher fiber intake is associated with lower blood pressure levels in patients with type 1 diabetes. Arch Endocrinol Metab 62:47–54 ArticleGoogle Scholar
- Wang S, Fang Y, Xu Y, Zhu B, Piao J, Zhu L, Yao L, Liu K, Wang S, Zhang Q, Qin L, Wu J (2022) The effects of different extraction methods on physicochemical, functional and physiological properties of soluble and insoluble dietary fiber from Rubus chingii Hu fruits. J Funct Foods 93:105081 ArticleGoogle Scholar
- Tejada-Ortigoza V, Garcia-Amezquita LE, Serna-Saldívar SO, Welti-Chanes J (2016) Advances in the functional characterization and extraction processes of dietary fiber. Food Eng Rev 8:251–271 ArticleGoogle Scholar
- Huang JY, Liao JS, Qi JR, Jiang WX, Yang XQ (2021) Structural and physicochemical properties of pectin-rich dietary fiber prepared from citrus peel. Food Hydrocoll 110:106140 ArticleGoogle Scholar
- Bashir S, Ahmad A, Abbasi KS, Akram Z (2022) Optimization of ultrasonic-assisted extraction of insoluble dietary fiber from wheat bran and its characterization. J Food Process Preserv 46(4):e16419 ArticleGoogle Scholar
- Yoshida BY, Prudencio SH (2020) Alkaline hydrogen peroxide improves physical, chemical, and techno-functional properties of okara. Food Chem 323:126776 ArticleGoogle Scholar
- Zhang W, Zeng G, Pan Y, Chen W, Huang W, Chen H, Li Y (2017) Properties of soluble dietary fiber-polysaccharide from papaya peel obtained through alkaline or ultrasound-assisted alkaline extraction. Carbohyd Polym 172:102–112 ArticleGoogle Scholar
- AOAC (2000). Official methods of analysis of the Association of Official Analytical Chemists (18 th ed.). Washington, DC.
- Larrauri JA, Rupérez P, Saura-Calixto F (1997) Effect of drying temperature on the stability of polyphenols and antioxidant activity of red grape pomace peels. J Agric Food Chem 45(4):1390–1393 ArticleGoogle Scholar
- Singleton VL, Rossi JA (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic 16(3):144–158 ArticleGoogle Scholar
- Brand-Williams W, Cuvelier ME, Berset CLWT (1995) Use of a free radical method to evaluate antioxidant activity. Lwt-Food Sci Technol 28(1):25–30 ArticleGoogle Scholar
- Niu Y, Li N, Xia Q, Hou Y, Xu G (2018) Comparisons of three modifications on structural, rheological and functional properties of soluble dietary fibers from tomato peels. LWT- Food Sci Technol 88:56–63 ArticleGoogle Scholar
- Chen H, Zhao C, Li J, Hussain S, Yan S, Wang Q (2018) Effects of extrusion on structural and physicochemical properties of soluble dietary fiber from nodes of lotus root. Lwt-Food Sci Technol 93:204–211 ArticleGoogle Scholar
- Koh PC, Leong CM, Noranizan MA (2014) Microwave-assisted extraction of pectin from jackfruit rinds using different power levels. Int Food Res J 21(5):2091 Google Scholar
- Liu X, Liu S, Xi H, Xu J, Deng D, Huang G (2019) Effects of soluble dietary fiber on the crystallinity, pasting, rheological, and morphological properties of corn resistant starch. LWT- Food Sci Technol 111:632–639 ArticleGoogle Scholar
- Alfredo VO, Gabriel RR, Luis CG, David BA (2009) Physicochemical properties of a fibrous fraction from chia (Salvia hispanica L.). Lwt-Food Sci Technol 42(1):168–173 ArticleGoogle Scholar
- Kuniak L, Marchessault RH (1972) Study of the crosslinking reaction between epichlorohydrin and starch. Starch-Stärke 24(4):110–116 ArticleGoogle Scholar
- Chau CF, Cheung PC (1999) Effects of the physico-chemical properties of three legume fibers on cholesterol absorption in hamsters. Nutr Res 19(2):257–265 ArticleGoogle Scholar
- Khatkar BS, Barak S, Mudgil D (2013) Effects of gliadin addition on the rheological, microscopic and thermal characteristics of wheat gluten. Int J Biol Macromol 53:38–41 ArticleGoogle Scholar
- Shao H, Zhang H, Tian Y, Song Z, Lai PF, Ai L (2019) Composition and rheological properties of polysaccharide extracted from tamarind (Tamarindus indica L.) seed. Molecules 24(7):1218 ArticleGoogle Scholar
- Zhu A, Tang L, Fu Q, Xu M, Chen J (2015) Optimization of alkali extraction of polysaccharides from foxtail millet and its antioxidant activities in vitro. J Food Biochem 39(6):708–717 ArticleGoogle Scholar
- Bendahou A, Dufresne A, Kaddami H, Habibi Y (2007) Isolation and structural characterization of hemicelluloses from palm of Phoenix dactylifera L. Carbohyd Polym 68(3):601–608 ArticleGoogle Scholar
- Ahmad A, Alkharfy KM, Wani TA, Raish M (2015) Application of Box-Behnken design for ultrasonic-assisted extraction of polysaccharides from Paeonia emodi. Int J Biol Macromol 72:990–997 ArticleGoogle Scholar
- Lin CB, Kai NY, Ali A (2018) Ultrasound assisted extraction of pectin from dragon fruit peels. J Eng Sci Technol 13:65–81 Google Scholar
- Moorthy IG, Maran JP, Muneeswari S, Naganyashree S, Shivamathi CS (2015) Response surface optimization of ultrasound assisted extraction of pectin from pomegranate peel. Int J Biol Macromol 72:1323–1328 ArticleGoogle Scholar
- Bagherian H, Ashtiani FZ, Fouladitajar A, Mohtashamy M (2011) Comparisons between conventional, microwave-and ultrasound-assisted methods for extraction of pectin from grapefruit. Chem Eng Process 50(11–12):1237–1243 ArticleGoogle Scholar
- Wang W, Wu X, Chantapakul T, Wang D, Zhang S, Ma X, Ding T, Ye X, Liu D (2017) Acoustic cavitation assisted extraction of pectin from waste grapefruit peels: a green two-stage approach and its general mechanism. Food Res Int 102:101–110 ArticleGoogle Scholar
- Patience NA, Schieppati D, Boffito DC (2021) Continuous and pulsed ultrasound pectin extraction from navel orange peels. Ultrason Sonochem 73:105480 ArticleGoogle Scholar
- Hu W, Ye X, Chantapakul T, Chen S, Zheng J (2020) Manosonication extraction of RG-I pectic polysaccharides from citrus waste: optimization and kinetics analysis. Carbohyd Polym 235:115982 ArticleGoogle Scholar
- Maran JP, Priya B (2015) Ultrasound-assisted extraction of pectin from sisal waste. Carbohyd Polym 115:732–738 ArticleGoogle Scholar
- Martinez-Solano KC, Garcia-Carrera NA, Tejada-Ortigoza V, García-Cayuela T, Garcia-Amezquita LE (2021) Ultrasound application for the extraction and modification of fiber-rich by-products. Food Eng Rev 13(3):524–543 ArticleGoogle Scholar
- Kazemi M, Khodaiyan F, Hosseini SS (2019) Eggplant peel as a high potential source of high methylated pectin: ultrasonic extraction optimization and characterization. LWT- Food Sci Technol 105:182–189 ArticleGoogle Scholar
- Nuerxiati R, Abuduwaili A, Mutailifu P, Wubulikasimu A, Rustamova N, Jingxue C, Aisa HA, Yili A (2019) Optimization of ultrasonic-assisted extraction, characterization and biological activities of polysaccharides from Orchis chusua D. Don (Salep). Int J Biol Macromol 141:431–443 ArticleGoogle Scholar
- Chen C, You LJ, Abbasi AM, Fu X, Liu RH (2015) Optimization for ultrasound extraction of polysaccharides from mulberry fruits with antioxidant and hyperglycemic activity in vitro. Carbohyd Polym 130:122–132 ArticleGoogle Scholar
- Minjares-Fuentes R, Femenia A, Garau MC, Meza-Velázquez JA, Simal S, Rosselló C (2014) Ultrasound-assisted extraction of pectins from grape pomace using citric acid: a response surface methodology approach. Carbohyd Polym 106:179–189 ArticleGoogle Scholar
- Guo X, Shang X, Zhou X, Zhao B, Zhang J (2017) Ultrasound-assisted extraction of polysaccharides from Rhododendron aganniphum: antioxidant activity and rheological properties. Ultrason Sonochem 38:246–255 ArticleGoogle Scholar
- Nguyen BMN, Pirak T (2019) Physicochemical properties and antioxidant activities of white dragon fruit peel pectin extracted with conventional and ultrasound-assisted extraction. Cogent Food Agric 5(1):1633076 ArticleGoogle Scholar
- Tang C, Wu L, Zhang F, Kan J, Zheng J (2022) Comparison of different extraction methods on the physicochemical, structural properties, and in vitro hypoglycemic activity of bamboo shoot dietary fibers. Food Chem 386:132642 ArticleGoogle Scholar
- Bhatt S, Gupta M (2022) Exploration of soluble dietary fiber extraction technique for enhancing physicochemical and structural properties of mango and pomegranate peel. Biomass Convers Biorefinery 14:1–16 Google Scholar
- Osorio-Esquivel O, Álvarez VB, Dorantes-Álvarez L, Giusti MM (2011) Phenolics, betacyanins and antioxidant activity in Opuntia joconostle fruits. Food Res Int 44(7):2160–2168 ArticleGoogle Scholar
- Viuda-Martos M, Ruiz-Navajas Y, Fernández-López J, Sendra E, Sayas-Barberá E, Pérez-Álvarez JA (2011) Antioxidant properties of pomegranate (Punica granatum L.) bagasses obtained as co-product in the juice extraction. Food Res Int 44(5):1217–1223 ArticleGoogle Scholar
- Díaz AB, Blandino A, Belleli C, Caro I (2014) An effective process for pretreating rice husk to enhance enzyme hydrolysis. Ind Eng Chem Res 53(27):10870–10875 ArticleGoogle Scholar
- de Camargo AC, Regitano-d’Arce MAB, Biasoto ACT, Shahidi F (2016) Enzyme-assisted extraction of phenolics from winemaking by-products: antioxidant potential and inhibition of alpha-glucosidase and lipase activities. Food Chem 212:395–402 ArticleGoogle Scholar
- Peng G, Gan J, Dong R, Chen Y, Xie J, Huang Z, Gu Y, Huang D, Yu Q (2021) Combined microwave and enzymatic treatment improve the release of insoluble bound phenolic compounds from the grapefruit peel insoluble dietary fiber. Lwt-Food Sci Technol 149:111905 ArticleGoogle Scholar
- Bolek S (2020) Olive stone powder: a potential source of fiber and antioxidant and its effect on the rheological characteristics of biscuit dough and quality. Innov Food Sci Emerg Technol 64:102423 ArticleGoogle Scholar
- Jiang G, Wu Z, Ameer K, Li S, Ramachandraiah K (2020) Particle size of ginseng (Panax ginseng Meyer) insoluble dietary fiber and its effect on physicochemical properties and antioxidant activities. Appl Biol Chem 63:1–10 ArticleGoogle Scholar
- Daou C, Zhang H (2014) Functional and physiological properties of total, soluble, and insoluble dietary fibres derived from defatted rice bran. J Food Sci Technol 51:3878–3885 ArticleGoogle Scholar
- Ma MM, Mu TH (2016) Effects of extraction methods and particle size distribution on the structural, physicochemical, and functional properties of dietary fiber from deoiled cumin. Food Chem 194:237–246 ArticleGoogle Scholar
- Liu CM, Liang RH, Dai TT, Ye JP, Zeng ZC, Luo SJ, Chen J (2016) Effect of dynamic high pressure microfluidization modified insoluble dietary fiber on gelatinization and rheology of rice starch. Food Hydrocoll 57:55–61 ArticleGoogle Scholar
- Wang C, Qiu WY, Chen TT, Yan JK (2021) Effects of structural and conformational characteristics of citrus pectin on its functional properties. Food Chem 339:128064 ArticleGoogle Scholar
- Zhang Y, Qi J, Zeng W, Huang Y, Yang X (2020) Properties of dietary fiber from citrus obtained through alkaline hydrogen peroxide treatment and homogenization treatment. Food Chem 311:125873 ArticleGoogle Scholar
- Gu M, Fang H, Gao Y, Su T, Niu Y, Yu LL (2020) Characterization of enzymatic modified soluble dietary fiber from tomato peels with high release of lycopene. Food Hydrocoll 99:105321 ArticleGoogle Scholar
- Xie F, Wang Y, Wu J, Wang Z (2016) Functional properties and morphological characters of soluble dietary fibers in different edible parts of Angelica keiskei. J Food Sci 81(9):C2189–C2198 ArticleGoogle Scholar
- Yang YY, Ma S, Wang XX, Zheng XL (2017) Modification and application of dietary fiber in foods. J Chem 2017:1–8 ArticleGoogle Scholar
- Korcz E, Kerényi Z, Varga L (2018) Dietary fibers, prebiotics, and exopolysaccharides produced by lactic acid bacteria: potential health benefits with special regard to cholesterol-lowering effects. Food Funct 9(6):3057–3068 ArticleGoogle Scholar
- Chau CF, Huang YL (2003) Comparison of the chemical composition and physicochemical properties of different fibers prepared from the peel of Citrus sinensis L. Cv. Liucheng. J Agric Food Chem 51(9):2615–2618 ArticleGoogle Scholar
- Ma S, Wang Z, Liu H, Li L, Zheng X, Tian X, Sun B, Wang X (2022) Supplementation of wheat flour products with wheat bran dietary fiber: purpose, mechanisms, and challenges. Trends Food Sci Technol 123:281–289 ArticleGoogle Scholar
- Huang YL, Chow CJ, Fang YJ (2011) Preparation and physicochemical properties of fiber-rich fraction from pineapple peels as a potential ingredient. J Food Drug Anal 19(3):4 Google Scholar
- Wang L, Xu H, Yuan F, Fan R, Gao Y (2015) Preparation and physicochemical properties of soluble dietary fiber from orange peel assisted by steam explosion and dilute acid soaking. Food Chem 185:90–98 ArticleGoogle Scholar
- Jiang G, Bai X, Wu Z, Li S, Zhao C, Ramachandraiah K (2021) Modification of ginseng insoluble dietary fiber through alkaline hydrogen peroxide treatment and its impact on structure, physicochemical and functional properties. LWT- Food Sci Technol 150:111956 ArticleGoogle Scholar
- Bouaziz A, Dridi D, Gargoubi S, Zouari A, Majdoub H, Boudokhane C, Bartegi A (2021) Study on the grafting of chitosan-essential oil microcapsules onto cellulosic fibers to obtain biofunctional material. Coatings 11(6):637 ArticleGoogle Scholar
- Zhao X, Zhu H, Chen J, Ao Q (2015) FTIR, XRD and SEM analysis of ginger powders with different size. J Food Process Preserv 39(6):2017–2026 ArticleGoogle Scholar
- Li N, Feng Z, Niu Y, Yu L (2018) Structural, rheological and functional properties of modified soluble dietary fiber from tomato peels. Food Hydrocoll 77:557–565 ArticleGoogle Scholar
- Du X, Wang L, Huang X, Jing H, Ye X, Gao W, Bai X, Wang H (2021) Effects of different extraction methods on structure and properties of soluble dietary fiber from defatted coconut flour. Lwt-Food Sci Technol 143:111031 ArticleGoogle Scholar
- Zhang Y, Liao J, Qi J (2020) Functional and structural properties of dietary fiber from citrus peel affected by the alkali combined with high-speed homogenization treatment. LWT- Food Sci Technol 128:109397 ArticleGoogle Scholar
- Fan X, Chang H, Lin Y, Zhao X, Zhang A, Li S, Feng S, Chen X (2020) Effects of ultrasound-assisted enzyme hydrolysis on the microstructure and physicochemical properties of okara fibers. Ultrason Sonochem 69:105247 ArticleGoogle Scholar
- Wu C, Teng F, McClements DJ, Zhang S, Li Y, Wang Z (2020) Effect of cavitation jet processing on the physicochemical properties and structural characteristics of okara dietary fiber. Food Res Int 134:109251 ArticleGoogle Scholar
- Yun L, Wu T, Liu R, Li K, Zhang M (2018) Structural variation and microrheological properties of a homogeneous polysaccharide from wheat germ. J Agric Food Chem 66(11):2977–2987 ArticleGoogle Scholar
- Xue Z, Ma Q, Guo Q, Santhanam RK, Gao X, Chen Z, Wang C, Chen H (2019) Physicochemical and functional properties of extruded dietary fiber from mushroom Lentinula edodes residues. Food Biosci 32:100452 ArticleGoogle Scholar
- Karaman E, Yılmaz E, Tuncel NB (2017) Physicochemical, microstructural and functional characterization of dietary fibers extracted from lemon, orange and grapefruit seeds press meals. Bioact Carbohydr Diet Fibre 11:9–17 ArticleGoogle Scholar
- Zhang J, Wang ZW (2013) Soluble dietary fiber from Canna edulis Ker by-product and its physicochemical properties. Carbohyd Polym 92(1):289–296 ArticleGoogle Scholar
- Kurek MA, Karp S, Wyrwisz J, Niu Y (2018) Physicochemical properties of dietary fibers extracted from gluten-free sources: quinoa (Chenopodium quinoa), amaranth (Amaranthus caudatus) and millet (Panicum miliaceum). Food Hydrocoll 85:321–330 ArticleGoogle Scholar
Author information
Authors and Affiliations
- Department of Biotechnology, Anand School of Engineering and Technology, Sharda University, Agra, 282007, India Divyani Panwar
- Food Biotechnology Research Laboratory, Department of Food Engineering and Technology, Sant Longowal Institute of Engineering and Technology, Longowal, 148106, Punjab, India Parmjit S. Panesar
- Department of Chemistry, Sant Longowal Institute of Engineering and Technology, Longowal, 148106, Punjab, India Harish K. Chopra
- Divyani Panwar
You can also search for this author in PubMed Google Scholar
You can also search for this author in PubMed Google Scholar
You can also search for this author in PubMed Google Scholar
Contributions
Divyani Panwar: conceptualization, investigation, formal analysis, data curation, writing—original draft preparation, writing—reviewing and editing.
Parmjit S. Panesar: conceptualization, resources, supervision, funding acquisition, writing—reviewing and editing.
Harish K. Chopra: resources and supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Dietary fiber was extracted from Citrus limetta peels using alkaline and ultrasound-assisted extraction techniques.
• Ultrasound-assisted enzymatic extraction method was optimized using Box-Behnken design.
• Extraction techniques affect proximate, technological, structural, and functional properties of fiber.
• Dietary fiber extracted using green technique exhibited superior thermal properties.
• Citrus peel dietary fiber could be utilized for the development of value-added functional products.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Panwar, D., Panesar, P.S. & Chopra, H.K. Green valorization approach of Citrus limetta peels by ultrasound-assisted enzymatic extraction for recovery of dietary fibers: Optimization, physicochemical, structural, functional, and thermal properties. Biomass Conv. Bioref. (2024). https://doi.org/10.1007/s13399-024-05963-x
- Received : 09 May 2024
- Revised : 11 July 2024
- Accepted : 16 July 2024
- Published : 05 August 2024
- DOI : https://doi.org/10.1007/s13399-024-05963-x
Share this article
Anyone you share the following link with will be able to read this content:
Get shareable link
Sorry, a shareable link is not currently available for this article.
Copy to clipboard
Provided by the Springer Nature SharedIt content-sharing initiative
Keywords
- Citrus peel
- Dietary fiber
- Ultrasound-assisted enzymatic extraction
- Box-Behnken design
- Alkaline extraction
- Characterization













